
Targeting the CD47-SIRPα axis is emerging as one of the most promising new cancer immunotherapy approaches seeking to target the innate immune response. A recent analysis reveals just how intense the global interest is and a growing list of stakeholders operating in this field.
Highest Stage Breakdown of CD47/SIRPα Molecules
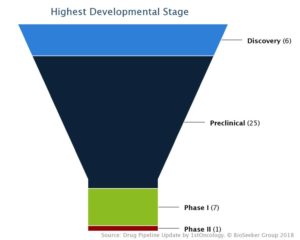
Most of the identified molecules are either anti-CD47 antibodies or SIRPα-Fc recombinant proteins. In healthy cells, CD47 serves as a “don’t eat me” signal by binding to the transmembrane SIRPα protein on phagocytic cells, preventing the engulfment of “self” by macrophages. In several cancer types, tumor cells overexpress CD47 to elude the immune system. Exploration of this property has been the driving force to attract hundreds of million of dollars in investments to companies like Tioma Therapeutics (now Arch Oncology), Surface Oncology and Forty Seven, the latter of which has just recently completed a $100M+ IPO. Deals in this area include the early adopter move in 2012 by Celgene securing Inhibrx’ anti-CD47 antibody in a $500 million dollar deal to the recent billion dollar plus agreement between OSE Immunotherapeutics and Boehringer Ingelheim.
On the patent front, it is evident that that the Synthon/Sanquin Blood Supply intellectual property rights hold some clout in the CD47 world following Forty Seven’s $47 million settlement and license agreement with Synthon. The license agreement also includes some far reaching concessions by Forty Seven in order for them to obtain freedom to operate. Moreover a recent patent analysis accessible in this product shows several new CD47/SIRPA bispecific/fusion-proteins, CD47 mimetics and additional, previously unknown, combination therapy strategies set forth along with methods for determining responsiveness to anti-cd47 agent
CD47/SIRPα Molecules Are In Development in Thirty Five Different Tumor Types
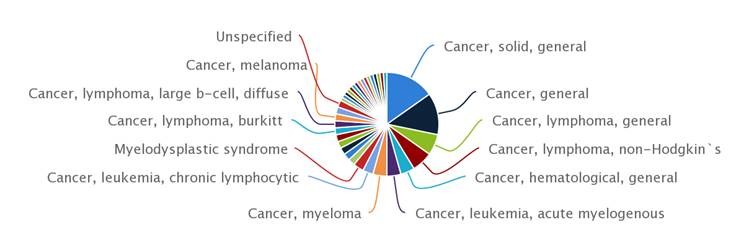
On the clinical development front, this report identifies fewer than ten molecules targeting the CD47-SIRPα axis which have made it into the clinic. So far with encouraging results, following the early termination of a Phase I/II trial of Tioma’s Ti-061 in late 2017.
Several of these trials also shed light on biomarker- and combination therapies of interest. On the horizon, this field will soon have another injection of candidate therapies with bispecific CD47 antibodies entering the clinic in late 2018 or early 2019.
The CD47/SIRPα Molecules Are Also Targeting Thirteen Other Targets, Including Bispecific Antibodies
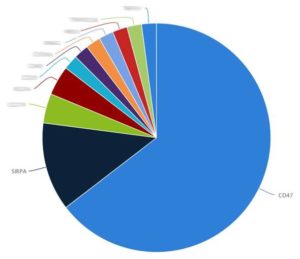
Moreover, targeting the CD47-SIRPα axis also has the potential to become the combination therapy of choice as has already been demonstrated via mediating longer survival in mice together with oncolytic virotherapy and enhancing phagocytic capacity when used together with Carisma Therapeutics’ CAR macrophages. Carisma Therapeutics themselves have just announced a $53 million series A round of financing in order to develop its novel CAR macrophage cellular immunotherapy and is estimating that their project(s) will enter into the clinic in 2019.
Schedule your 30 min Free 1stOncology Demo!
Discover why more than 1,500 members use 1stOncology™ to excel in:
Early/Late Stage Pipeline Development - Target Scouting - Clinical Biomarkers - Indication Selection & Expansion - BD&L Contacts - Conference Reports - Combinatorial Drug Settings - Companion Diagnostics - Drug Repositioning - First-in-class Analysis - Competitive Analysis - Deals & Licensing
Schedule Your 30 min Free Demo!