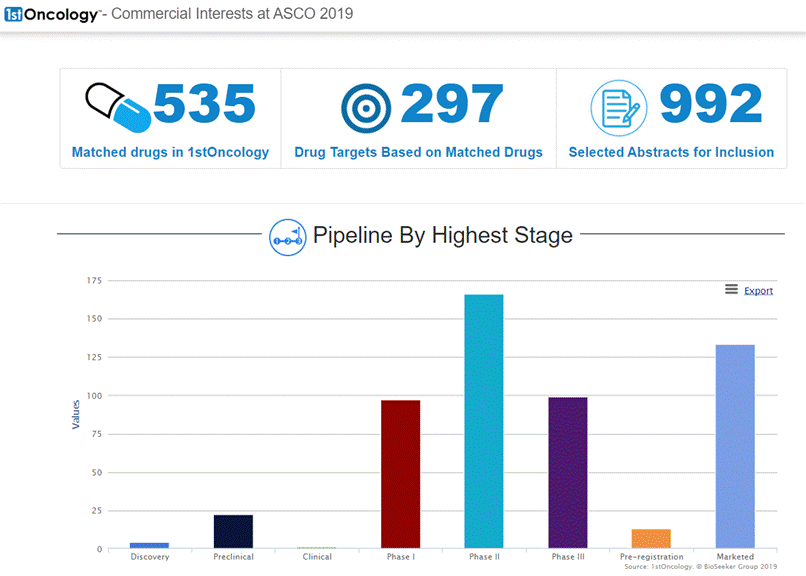
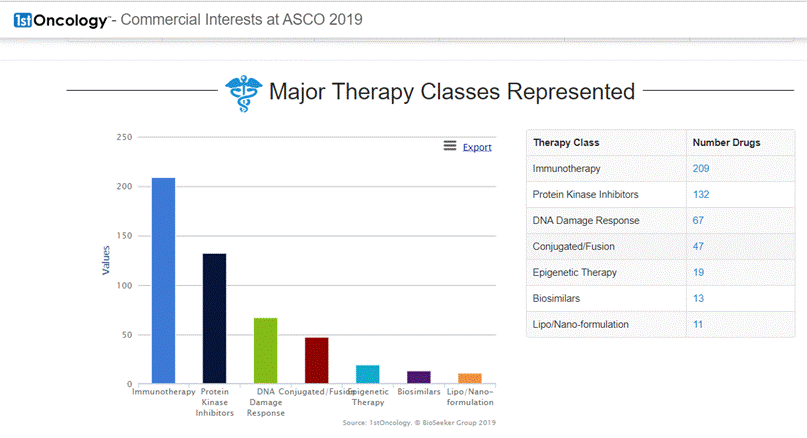
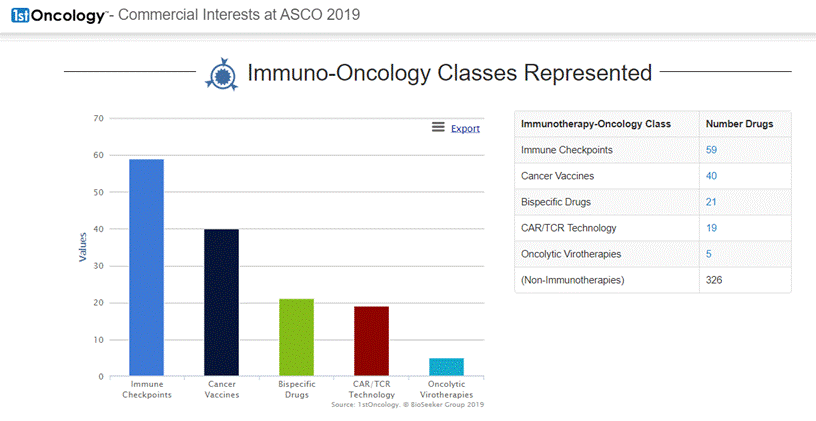
On June 11, 2019 DelMar Pharmaceuticals, Inc. (NASDAQ: DMPI) ("DelMar" or the "Company"), a biopharmaceutical company focused on the development and commercialization of new cancer therapies, reported that it has adjusted certain pricing information and key dates for its previously announced rights offering (Press release, DelMar Pharmaceuticals, JUN 11, 2019, View Source [SID1234536994]). The subscription period for the rights offering will now expire at 5:00 PM Eastern time on June 25, 2019, unless extended by the Company.
Schedule your 30 min Free 1stOncology Demo!
Discover why more than 1,500 members use 1stOncology™ to excel in:
Early/Late Stage Pipeline Development - Target Scouting - Clinical Biomarkers - Indication Selection & Expansion - BD&L Contacts - Conference Reports - Combinatorial Drug Settings - Companion Diagnostics - Drug Repositioning - First-in-class Analysis - Competitive Analysis - Deals & Licensing
Schedule Your 30 min Free Demo!
The unit pricing remains $1,000 per unit, consisting of one share of Series C Convertible Preferred Stock with a stated value of $1,000 (and immediately convertible into shares of DelMar’s common stock) and warrants to purchase DelMar’s common stock. The Series C Convertible Preferred Stock conversion price will now be $3.10 and each unit will now consist of 209 warrants to purchase DelMar’s common stock at an adjusted exercise price of $3.10 per share. The warrants will still be exercisable for five (5) years after the date of issuance and shall be redeemable as described in the preliminary and final prospectus, when available.
If exercising subscription rights through a broker, dealer, bank or other nominee, rights holders should promptly contact their nominee and submit subscription documents and payment for the units subscribed for in accordance with the instructions and within the time period provided by such nominee. The broker, dealer, bank or other nominee may establish a deadline before June 25, 2019, by which instructions to exercise subscription rights, along with the required subscription payment, must be received.
All record holders of rights that wish to participate in the rights offering must deliver a properly completed and signed subscription rights statement, together with payment of the subscription price for both basic subscription rights and any over subscription privilege election for delivery no later than 5:00 PM Eastern Time on June 25, 2019 to the Subscription Agent:
By mail:
By hand or overnight courier:
Broadridge Corporate Issuer Solutions, Inc.
Attn: BCIS Re-Organization Dept.
P.O. Box 1317
Brentwood, New York 11717-0693
(888) 789-8409 (toll free)
Broadridge Corporate Issuer Solutions, Inc.
Attn: BCIS IWS
51 Mercedes Way
Edgewood, New York 11717
(888) 789-8409 (toll free)
Under the rights offering, DelMar distributed one non-transferable subscription right for each share of common stock and each participating warrant held on the record date. The subscription rights are exercisable for up to an aggregate of $1.9 million of units on a pro rata basis if subscriptions are received in excess of that threshold.
Holders who fully exercise their basic subscription rights will be entitled, if available, to subscribe for an additional amount of units that are not purchased by other holders, on a pro rata basis and subject to the $1.9 million aggregate offering threshold and other ownership limitations.
DelMar has engaged Maxim Group LLC and Dawson James Securities Inc. as co-dealer-managers in the rights offering. Questions about the rights offering or requests for copies of the preliminary and final prospectuses, when available, may be directed to Maxim Group LLC at 405 Lexington Avenue, New York, NY 10174, Attention Syndicate Department, or via email at [email protected] or telephone at (212) 895-3745.
A registration statement relating to these securities has been filed with the Securities and Exchange Commission (the "SEC") and became effective on May 28, 2019, and is available on the SEC’s website located at View Source Additionally, a post-effective amendment to the registration statement was filed on June 10, 2019 for pricing and other adjustments discussed above. The rights offering is being made only by means of a written prospectus. A copy of the prospectus for the rights offering may be obtained, when available, from Maxim Group LLC, 405 Lexington Avenue, New York, NY 10174, Attention Syndicate Department, email: [email protected] or telephone (212) 895-3745. Investors may also obtain these documents at no cost by visiting the SEC’s website at View Source
This press release does not constitute an offer to sell or the solicitation of an offer to buy these securities, nor will there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.