
The 2018 Nobel Prize in Physiology or Medicine has been awarded jointly to James P. Allison and Tasuku Honjo for their almost 30 year old discovery of cancer therapy by inhibition of negative immune regulation. It was in the 1990s, when Dr James P. Allison’s laboratory at the University of California, Berkeley studied the T-cell protein CTLA-4. He was one of several scientists who had made the observation that CTLA-4 functions as a brake on T cells. In 1992, a few years before Allison’s discovery, Tasuku Honjo discovered PD-1, another protein expressed on the surface of T-cells. His research showed that PD-1, similar to CTLA-4, functions as a T-cell brake, but operates by a different mechanism.
Schedule your 30 min Free 1stOncology Demo!
Discover why more than 1,500 members use 1stOncology™ to excel in:
Early/Late Stage Pipeline Development - Target Scouting - Clinical Biomarkers - Indication Selection & Expansion - BD&L Contacts - Conference Reports - Combinatorial Drug Settings - Companion Diagnostics - Drug Repositioning - First-in-class Analysis - Competitive Analysis - Deals & Licensing
Schedule Your 30 min Free Demo!
Fast forwarding to today and cancer immunotherapy is the established 4th pillar of cancer therapy and encompasses a highly diverse portfolio of treatment strategies from the early interferon-α/interleukin-2 therapies to the current progress of immune-checkpoint inhibitors and CAR-T therapies to make the immune system fight cancer.
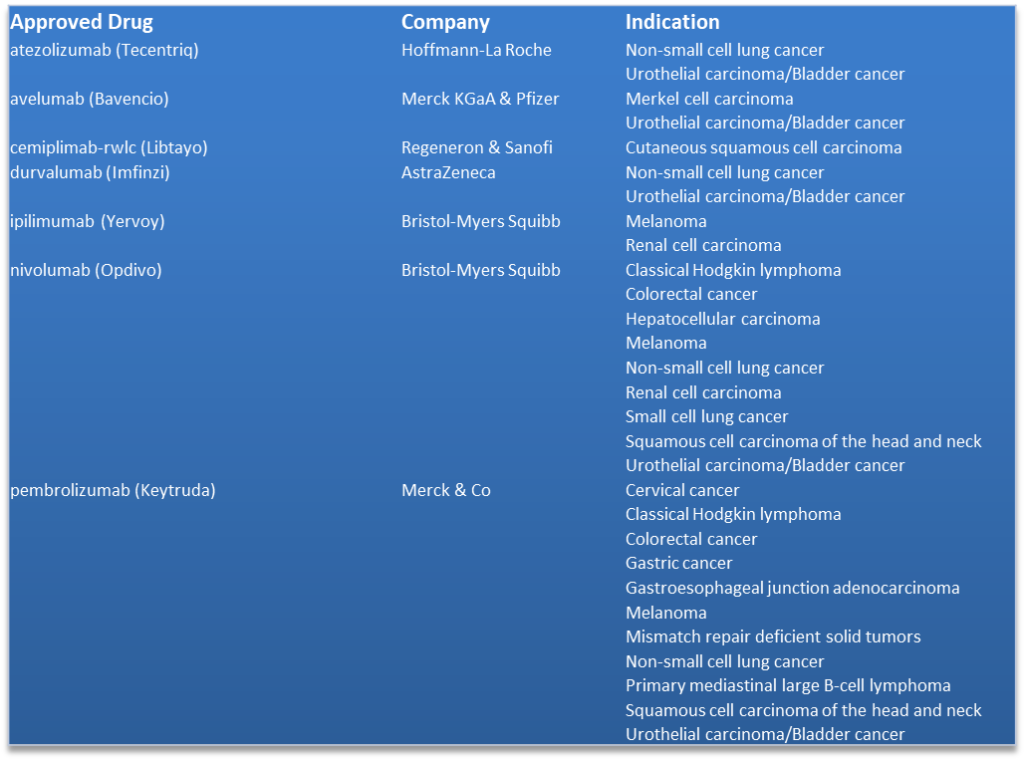
The first approved immune-checkpoint inhibitor was Bristol-Myers Squibb’s ipilimumab (Yervoy) back in 2011 for the treatment of adult patients with previously-treated advanced melanoma. Then followed the approval of Opdivo (nivolumab) and Keytruda (pembrolizumab) in rapid succession. Currently there are seven approved immune-checkpoint inhibitors for the treatment of sixteen different tumor types, see table below. Just last Friday Regeneron and Sanofi reported that the U.S. Food and Drug Administration (FDA) has approved Libtayo (cemiplimab-rwlc) for the treatment of patients with metastatic cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC who are not candidates for curative surgery or curative radiation.
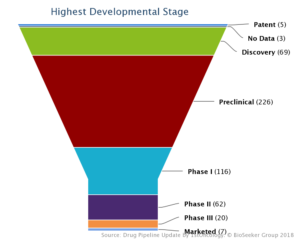
These approved drugs are just the tip of the iceberg with more than 500 immune-checkpoint drugs being developed world-wide for the treatment of cancer. No less than twenty of these are in late stage development.
The event of immune-checkpoint drugs such as nivolumab (Opdivo) and pembrolizumab (Keytruda) have dramatically changed the possibility to harness the power of the immune system in fighting cancer not only as a monotherapy but, maybe more importantly, also as a combination therapy.