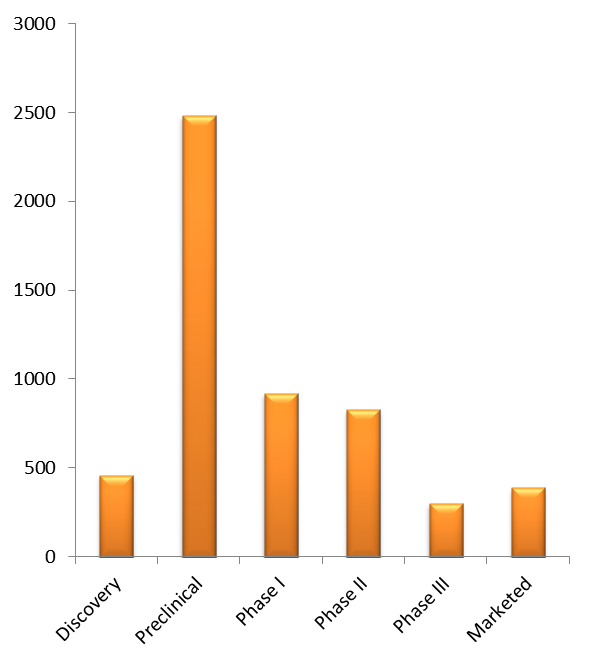
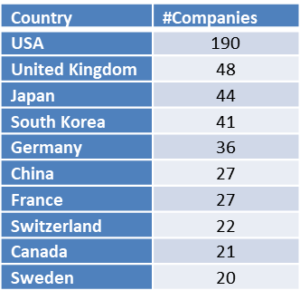
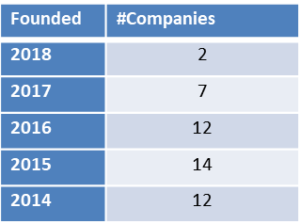
On November 6, 2018 Iovance Biotherapeutics, Inc. (NASDAQ: IOVA), a biotechnology company developing novel cancer immunotherapies based on tumor-infiltrating lymphocyte (TIL) technology, reported its third quarter 2018 financial results and provided a corporate update (Press release, Iovance Biotherapeutics, NOV 6, 2018, View Source;p=RssLanding&cat=news&id=2375637 [SID1234530753]).
Schedule your 30 min Free 1stOncology Demo!
Discover why more than 1,500 members use 1stOncology™ to excel in:
Early/Late Stage Pipeline Development - Target Scouting - Clinical Biomarkers - Indication Selection & Expansion - BD&L Contacts - Conference Reports - Combinatorial Drug Settings - Companion Diagnostics - Drug Repositioning - First-in-class Analysis - Competitive Analysis - Deals & Licensing
Schedule Your 30 min Free Demo!
"We have made tremendous progress in the last several months. Following our recent end of Phase 2 meeting with the FDA, we released information about the meeting outcome and our US registration path, receipt of RMAT designation for metastatic melanoma, an abbreviated set of our latest clinical data, and conducted a successful round of financing. The company is now in a very strong financial position which will allow us to pursue our registration program to commercialize our TIL therapy," said Dr. Maria Fardis, Ph.D., MBA, president and chief executive officer of Iovance Biotherapeutics. "We intend to recruit a new cohort of patients in the C-144-01 study to support registration of lifileucel, build a commercial manufacturing facility as well as a commercial team to support our plans to bring lifileucel to patients, while we continue advancing our existing clinical programs and expand our TIL therapy into new indications."
Recent Achievements
Regulatory
Iovance received the Regenerative Medicine Advanced Therapy (RMAT) designation for lifileucel, the company’s adoptive cell therapy using its TIL technology for the treatment of patients with metastatic melanoma from the U.S. Food and Drug Administration (FDA).
Iovance held an end of Phase 2 meeting with FDA during which the agency acknowledged that a single-arm cohort as part of the C-144-01 study could be supportive of initial registration and conduct of a randomized Phase 3 trial in the patient population being enrolled may not be feasible.
Clinical
Enrollment in Cohort 2 of the global Phase 2 lifileucel metastatic melanoma study, C-144-01, reached the predefined sample size and was therefore closed.
— As announced today, new data from Cohort 2 will be presented in a poster and as an oral presentation at the Society for Immunotherapy of Cancer (SITC) (Free SITC Whitepaper) 33rd Annual Meeting in Washington, D.C. on November 10-12. The presentation will include 47 consecutively dosed patients with an objective response rate (ORR) of 38%, with a median duration of response (DOR) of 6.4 months and range of 1.3+ to 14+ months. The ORR includes one complete response and 17 partial responses, four of which are unconfirmed and pending patient’s upcoming second assessments. Patients in the study had a mean of 3.3 prior systemic therapies and all the patients had received anti-PD-1 immunotherapy.
— The most common treatment emergent adverse events observed in this cohort to date include chills, febrile neutropenia, anaemia, decreased platelet count, pyrexia, and hypophosphataemia. There were two grade 5 events reported.
— Patient enrollment in a new cohort, Cohort 4, will be initiated in early 2019. This will be a single-arm cohort for registration in metastatic melanoma in a patient population that is post PD-1 blocking antibody and, if BRAF mutation positive, a BRAF inhibitor or BRAF inhibitor with MEK inhibitor. Iovance expects to fully enroll the necessary patients into this cohort by late 2019/early 2020. Cohort 4 is expected to enroll 80-100 patients. The primary endpoint for the study is ORR as determined by Blinded Independent Central Review (BIRC).
Patient dosing continues in the C-145-04 study for cervical carcinoma. The company recently dosed its first patient in Europe. This study design is based on a Simon’s two-stage design. The first stage has been completed and enrollment in the study continues with target enrollment of 47. Preliminary data for 15 patients yielded an ORR of 27% with an early look at the DOR ranging from 2.4 to 2.5+ months. Patients in the study had a median of five prior therapies. The safety findings from this study remain consistent with previous reports. The protocol for this study has been amended to limit the number of prior therapies to no more than three and to exclude patients who have been treated with prior immunotherapy. Iovance anticipates providing an update on this study at an upcoming medical meeting in 2019.
In the C-145-03 study for head and neck cancer, to date, preliminary data for 13 patients has yielded an ORR of 31% with the DOR ranging from 2.8 to 7.6 months. The safety findings from this study is also consistent with previous reports. Patients in the study had a median of three prior therapies.
For the study in NSCLC, IOV-LUN-201, in collaboration with MedImmune, the company amended the protocol to eliminate the TIL monotherapy cohort and patients will now be enrolled for treatment with LN-145 and durvalumab. There are currently nine sites active for this trial.
The study in PD-1 naïve melanoma and head and neck patients with TIL in combination with pembrolizumab, and LN-145 as monotherapy in NSCLC patients (IOV-COM-202) is open to enrollment with two sites active.
As of October 2018, Iovance has expanded to over 90 clinical sites for its five company-sponsored studies.
Manufacturing
Iovance announced a new three-year Manufacturing Services Agreement with MaSTherCell S.A., a cell therapy-dedicated Contract Development and Manufacturing Organization (CDMO). MaSTherCell will manufacture TIL for Iovance’s European late-stage clinical trials in its commercial-ready cGMP manufacturing suites and increases Iovance’s capacity for manufacturing TIL in Europe.
Research
Under a collaboration with Ohio State University, Iovance has developed a product candidate called peripheral blood lymphocytes (PBLs). A clinical program to administer PBLs in chronic lymphocytic leukemia (CLL) patients is expected to begin in 2019.
Data from PD-1 selected TIL, one of the next generation TIL products at Iovance, will also be presented at SITC (Free SITC Whitepaper).
Corporate
In October 2018, the company closed an underwritten public offering of 25,300,000 shares of its common stock at a public offering price of $9.97 per share, before underwriting discounts. The shares sold at closing included 3,300,000 shares issued upon the exercise in full by the underwriter of its option to purchase additional shares at the public offering price less the underwriting discount. The net proceeds from the offering, after deducting the underwriting discounts and commissions and other offering expenses payable by the company, were $236.6 million.
Two U.S. patent applications covering therapeutic methods based upon Generation 2 manufacturing, which was developed at Iovance, were recently allowed.
Third Quarter 2018 Financial Results
Net loss for the third quarter ended September 30, 2018 was $33.8 million, or $0.36 per share, compared to a net loss of $22.1 million, or $0.35 per share for the same period ended September 30, 2017.
Research and development expenses were $27.9 million for the third quarter ended September 30, 2018, an increase of $11.3 million compared to $16.7 million for the third quarter ended September 30, 2017. The increase was primarily attributable to a $4.8 million increase in clinical trial costs due to; higher patient enrollment and an increase in the number of sites in the clinical trial of lifileucel for the treatment of metastatic melanoma, increased enrollment in the cervical and head and neck LN-145 clinical trials and the initiation of clinical trials in 2018 for new indications. Further, payroll and related expenses, including stock-based compensation expenses increased by $4.4 million due to a higher number of full time employees and dedicated consultants as the company expanded its internal research efforts and clinical development programs. In addition, research and alliance costs increased by $1.4 million for clinical trials run by Iovance’s alliance partners and new research initiatives and $0.7 million for the expansion of manufacturing capacity at the company’s Clinical Manufacturing Organizations (CMOs).
General and administrative expenses were $7.1 million for the third quarter ended September 30, 2018, an increase of $1.4 million compared to $5.7 million for the third quarter ended September 30, 2017. The increase was primarily attributable to a $1.5 million increase in stock-based compensation expenses due to an increase in the number of full time employees and higher stock prices during the quarter as compared to the same period in 2017.
Nine Months Ended September 30, 2018 Financial Results
Net loss for the nine months ended September 30, 2018 was $91.0 million, or $1.01 per share, compared to a net loss of $66.2 million, or $1.06 per share for the same period ended September 30, 2017.
Research and development expenses were $72.4 million for the nine months ended September 30, 2018, an increase of $21.5 million compared to $50.9 million for the same period ended September 30, 2017. The increase was primarily attributable to a $11.9 million increase in the company’s clinical trial costs for ongoing and newly initiated studies and a $10.1 million increase in payroll and related expenses, including stock-based compensation expenses, for a higher number of full time employees and expenses for services performed by third parties in support of the company’s clinical studies. Further, research and research alliance costs increased by $1.1 million as Iovance expanded its research efforts and the number of clinical development programs run by its collaborators. These increases were partially offset by a $1.5 million decrease in manufacturing costs due to higher costs in 2017 related to technical transfer activities.
General and administrative expenses were $20.9 million for the nine months ended September 30, 2018, an increase of $5.0 million compared to $15.9 million for the same period ended September 30, 2017. The increase was primarily attributable to a $4.3 million increase in payroll and related expenses, including stock-based compensation expenses, due to a higher number of full time employees and higher stock prices during 2018 and a $0.6 million increase in professional service and legal expenses.
At September 30, 2018, the company held $260 million in cash, cash equivalents, and short-term investments compared to $276.1 million at June 30, 2018. During the third quarter the company used $28.2 million for operating-related activities and received $12.1 million of proceeds from the exercise of warrants and stock options. In October 2018 the company received $236.6 in net proceeds from the issuance of common stock. The company anticipates that the year-end balance of cash, cash equivalents and short-term investments may be between $460 to $465 million.
Webcast and Conference Call
Iovance will host a conference call today at 4:30 p.m. ET to discuss these third quarter 2018 results and provide a corporate update. The conference call dial-in numbers are 1-844-646-4465 (domestic) or 1-615-247-0257 (international). The conference ID access number for the call is 8718039. The live webcast can be accessed under "News & Events" in the "Investors" section of the company’s website at View Source or you may use the link: View Source
A replay of the call will be available from November 6, 2018 at 7:30 p.m. ET to November 13, 2018 at 8:30 p.m. ET. To access the replay, please dial 1-855-859-2056 (domestic) or 1-404-537-3406 (international) and reference the access code 8718039. The archived webcast will be available for thirty days in the Investors section of Iovance Biotherapeutics’ website at View Source.